How the elements are made
The manufacture of all the elements of the periodic table is the result of nucleosynthesis within stars, successively heavier elements are created by combining the atoms that form the nuclei of lighter elements. The most abundant element in the observable Universe is Hydrogen, which occurs in three “flavours” or isotopes, the simplest form, Protium, consisting of a single proton orbited by a single electron.
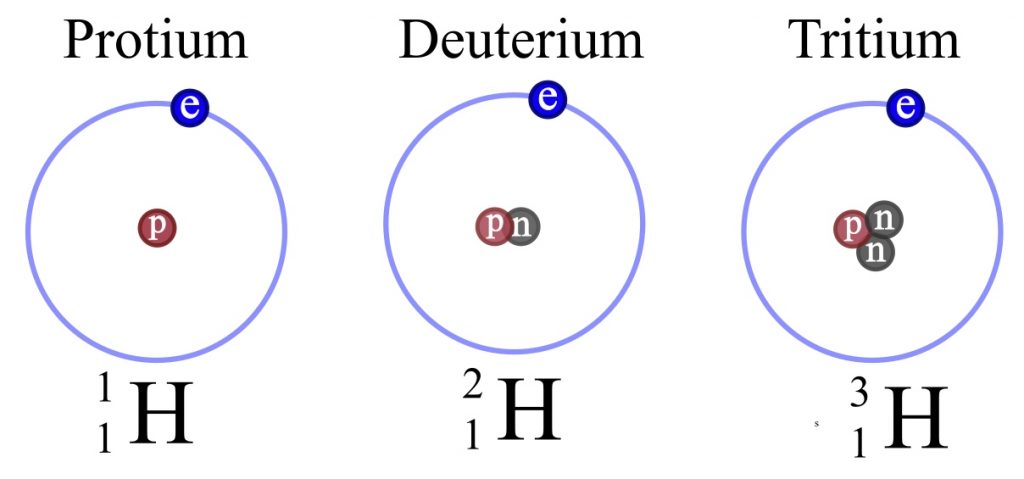
Fig 1: The three isotopes, or flavours, of Hydrogen, the most abundant element in the observable Universe.
It is currently believed that as the Universe began to cool, following the big bang and expansion, forces started to form quarks and gluons together, this formed the Protons, electrons and Neutrons we see making up the Observable Universe. As this matter continued to cool, other forces, such as the strong and weak Nuclear forces, began to make themselves felt, this forced particles together and within a very short period the majority of the particles in that early Universe were formed into the three isotopes of Hydrogen and also, Helium, the second most abundant element in the Universe.
Helium, like Hydrogen, occurs is more than 1 natural isotope, or flavour;

Figure 2: The two naturally occurring isotopes of Helium.3He consists of 2 Protons and a Neutron, 4He is 2 Protons and 2 Neutrons. Both have a pair of electrons orbiting under normal circumstances. Ionisation can add or remove electrons which changes the electrical properties of the atom.
As the hydrogen and helium formed in the early universe some parts had a greater density than others. As gravity built due to the increased mass this hydrogen and helium began to form large clouds, momentum likely caused motions within the clouds and also forced them to rotate. Eventually some of these clouds became large enough that they were drawn inward by their own gravity, becoming ever denser at the heart of the cloud. As the atoms were drawn in they jostled together, something they don’t like to do, this friction created infra-red radiation (heat) that put increasing amounts of energy into the atoms, increasing the velocity they moved within the cloud.
Over a period of time the core of the cloud became dense enough and hot enough that the motion of the atoms was able to overcome the strong nuclear forces, which normally keeps atoms well away from each other, this caused the atoms to “crash” into each other, these collisions caused the atoms to “fuse” together, release energy and create heavier atoms. These early stars were true behemoths, hundreds of times the mass of our Sun and the majority of stars we see in the Universe today. However, the fusion of hydrogen nuclei, single proton nuclei, to form atoms consisting of two protons (Helium) is the method by which all stars, those in the early Universe, and today, produce energy and start the process of nucleosynthesis.
All stars produce heavier elements but the types of elements they produce is dependent on their mass and how they end their lives. The more massive stars produce heavier elements throughout their lives, but the truly heavy elements, those above 56Ir (Iron) are only created during Supernova events.
Main sequence stars, those that are producing their energy via hydrogen fusion can be any mass, ranging from the smallest stars, that may live for trillions of years, to stars like the Sun, which may live for 10 billion years to the Supergiants, like Betelgeuse, which are 10 to 20 times the mass of the Sun and only live for an estimated 10-15 million years, to stars like Eta Carina that may be more than 100 times the mass of the Sun, and only live for around 1 million years.
As the Sun ages it will become a Red Giant, not because it will increase in mass, actually the opposite is true, but because as it ages, the core fills up with Helium, slowing the fusion process in its core, resulting in less energy production, resulting in gravity increasing it’s grip and squeezing it, the core, ever denser, at the same time the outer layers of the Sun, in fact all main sequence stars, will cool and feel less of gravity’s grip, so they swell. The core will heat as the atoms are squeezed together, the result will be Helium fusion within the core, producing Carbon atoms , at a temperature of around 100 million degrees Kelvin, this period for the Sun may last about 1 billion years, but the Sun lacks sufficient mass to fuse Helium and Carbon.
Heavier starts will continue this process, as each new “core”, which is in fact a layer atop the true core, fuses elements to make heavier elements, gravity continues to make itself felt and crushing the core more. Obviously, the speed with which this process occurs depends on the mass of the star.
For Supergiant stars, in the mass range of 10-20 solar masses, the burning of helium to produce heavier elements then continues for around a million years. The alpha-process (Alpha particles (radiation) are actually Helium nuclei) then combines helium with carbon to produce heavier elements, but only those with an even number of protons.
The elements grow in this order;
- 12Carbon + 4Helium = 16Oxygen
- 16Oxygen + 4Helium = 20Neon
- 20Neon + 4Helium = 24Magnsium
- 24Magnesium + 4Helium = 28Silicon
- 28Silicon + 4Helium = 32Sulfur
- 32Sulfur + 4Helium + 4Helium = 40Argon
- 40Argon + 4Helium = 40Calcium
- 40Calcium + 4Helium + 4Helium = 48Titanium
- 48Titanium + 4Helium = 52Chromium
- 52Chromium + 4Helium = 56Iron
There are other fusion pathways that create the elements with odd numbers of protons. 56Iron has such a tightly bound nucleus that conventional fusion cannot proceed to create heavier elements beyond this point. Without fusion in the core, there is insufficient energy within the core to prevent the immense gravity of the star causing it to undergo a core collapse – the star has become a Type II Supernova.
Physicist Lawrence Krauss has suggested that it may only take 100,000 years for the carbon to fuse into oxygen, perhaps only 10,000 years for the oxygen to fuse into silicon, and perhaps only one day for the silicon to fuse into iron, heralding the collapse of the star.
When the star’s core collapses, and it becomes a Type II Supernova, the energy released is sufficient that 56Iron atoms can be fused with additional elemental nuclei and allow the death of the star to seed the surrounding environment with every element we know of.
Astronomer Carl Sagan in the TV series “Cosmos” noted, “We are made of star-stuff.” Krauss agreed, stating that “every atom in your body was once inside a star that exploded…The atoms in your left hand probably came from a different star than in your right hand, because 200 million stars have exploded to make up the atoms in your body.”
